Section: New Results
Other detection approaches
Illumination modeling and chromophore identification in dermatological images for skin disease analysis
Participants : Zhao Liu, Josiane Zerubia [contact] .
This work is part of the LIRA Skin Care Project, which includes four key partners: Philips R&D [http://www.research.philips.com ], CWI (Netherlands) [http://www.cwi.nl ], Inria (France), and Fraunhofer Institutes (Germany) [http://www.fraunhofer.de/en.html ].
Chromophore identification, illumination modeling, skin disease analysis, dermatology
Skin color is an important characteristic for the accurate diagnosis and grading of cutaneous lesions by experienced dermatologists in clinical practice. However, the visual perception of skin color is not only a function of the major chromophores (melanin and hemoglobin) underneath the skin surface, but is also affected by external illumination and the spectral responses of imaging detectors. Skin color representation in a specific color space (e.g. RGB and its transformations) is not a genuine physical quantity. It sometimes fails to provide precise information about the concentrations of cutaneous chromophores, and is easily influenced by external imaging factors. As a result, conventional colorimetry may not properly describe the underlying histological content of skin, and hence tends to yield less trustworthy results when applied directly for skin disease analysis.
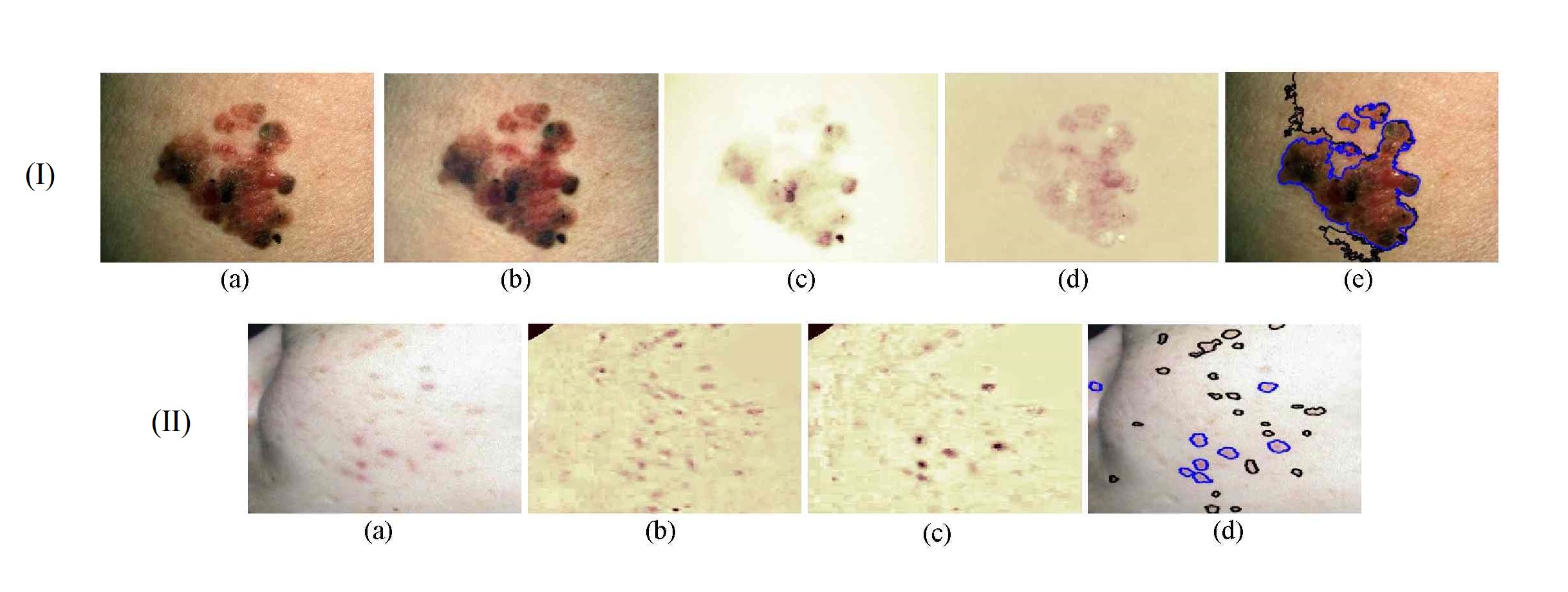
Building on a previous study that considered human skin as a diffuse reflectance surface, our work models human skin as having specular and diffuse reflectance, leading to a novel illumination correction method. Based on this method, we have developed a new scheme for chromophore identification from dermatological photographs. The algorithm has three steps. First, specular reflectance is separated from diffuse reflectance in the original skin images through specular pixel localization and image interpolation using a nonlinear weighted averaging process. Second, the resultant diffuse reflectance component is decomposed into a base layer and a detail layer. The base layer, representing low-frequency illumination and shading effects, is approximated by polynomial curve fitting using an initial illumination map using an adaptive bilateral filter as a prior. The detail layer, primarily containing high-frequency chromophore reflectance, can then be calculated by subtracting the base layer from the corresponding diffuse spectral band in logarithmic form. Finally, by incorporating knowledge of chromophore absorption characteristics, melanin and hemoglobin densities are identified using the detail layers from different spectral channels [11] .
For algorithm evaluation, the method was applied to two skin disease analysis problems: computer-aided melanoma diagnosis [11] and automatic acne detection [12] . For melanoma diagnosis, 201 conventional RGB skin lesion images (62MMs, 139 benign nevi (BN)) were collected from free public databases (http://www.dermquest.com/ , http://www.dermis.net/ ) to form an experimental data set. Figure 13 -(I) shows an example of a superficial spreading melanoma with obvious horizontal shading effects, and the corresponding experimental results. It is clear that the proposed algorithm successfully removed the imaging artifacts from the original skin lesion photographs.
For acne detection, a set of 50 challenging images were tested as a qualitative evaluation to demonstrate the usefulness of the proposed method. Automatic acne segmentation is performed using an MRF model based on chromophore descriptors. Figure 13 -(II) shows one acne example captured in an uncontrolled environment from a free public database (http://www.dermnetnz.org/ ). The detected acne areas are highly consistent under visual inspection, and the inflammatory acne can be distinguished from hyperpigmentation by comparing the average values of the melanin and hemoglobin indices.
|